We are conducting translational research by working with our zebrafish model of human disease, testing novel therapies in the lab, and developing new diagnostic methods. Our multi-disciplinary, highly collaborative, “bench-to-bedside” approach is to keep the research problems addressed as clinically relevant as possible, by which the knowledge generated in the animal model can be moved to the patient to the greatest possible extent.
Since 2009 we have been conducting translational research in the field of clinical neuroscience using zebrafish and human models in the Department of Neurology at the University Hospital Zurich. Being interested in the eyes and the brain, we focus on research questions with both physiological and behavioral aspects. We look at different sensory-motor systems, namely visual and vestibular ocular motor systems, and their interaction in diseased and healthy subjects. Particularly, we are interested in the pathological mechanisms of the infantile nystagmus syndrome (INS, formerly called congenital nystagmus), an ocular motor disorder characterized by several typical nystagmus waveforms. INS is often associated with visual pathway abnormalities such as present in albinism (temporal retinal misprojections), isolated achiasmia (non-decussating retinal-fugal fiber syndrome), and hypochiasma. To date, restrictions inherent to human research and the absence of a handy animal model have impeded efforts to identify the underlying mechanism of INS. Thus, we employ an achiasmatic zebrafish mutant belladonna, which displays INS-like spontaneous nystagmus, as a model organism to study INS. The long-term goal of this project is to expand our knowledge of INS, propose new treatment avenues for INS, and increase our understanding of the ocular motor systems in vertebrates in general.
Previous and current research
We have been employing zebrafish as a model organism to study visuomotor feedback control in the healthy and pathological ocular motor system. Specifically, our recent discovery of an achiasmatic zebrafish mutant with spontaneous nystagmus promises new insights into the etiology of INS. The mutants display not only INS-like ocular motor instabilities, but also other symptoms common to human INS such as a reversed optokinetic response and problems in locomotor functioning, observable as looping (swimming in circles). To further validate our animal model for INS linked to optic nerve misrouting and to test our working hypothesis of INS as a developmental disorder, we have been focusing on addressing following questions in zebrafish:
1) From a sensory aspect: How can a reversed sensory input due to visual pathway error change the feedback of the ocular motor system?
2) From a motor aspect: How is gaze stabilization influenced by an alteration in the ocular feedback? What brain plasticity/adaptation processes (e.g., long-term tuning of the neural integrator) take place as a result of a constantly high internal gain?
3) INS patients have a reduced postural balance and sometimes experience dizziness and oscillopsia, suggesting that the performance of the vestibular system is also altered. We therefore aim to investigate the influence of the impaired optokinetic system in zebrafish with retinotectal misprojections on the vestibulo-ocular system during development and in adulthood.
Based on the supposed link between abnormal retinotectal projections and ocular motor instabilities, we plan to develop a diagnostic algorithm to screen for aberrant visual pathways in humans solely based on eye movement recordings. Additionally, we will test optical and other treatment methods. Finally, using zebrafish with spontaneous nystagmus, we currently perform a screening for potential new drugs for INS and test existing neurological drugs that have not yet been tested in INS patients in order to discover new pharmacological treatment options for INS in humans.
Future research
(1) To directly test our hypothesis, stating that visual pathway abnormality plays a role in the etiology of INS, we will ask if INS can be induced in adult wild-type fish by surgically disconnecting the optic nerves followed by guiding the regeneration of the nerves to the wrong hemisphere. (2) We will develop a novel associate and non-associate learning paradigm in zebrafish, aiming to study cognitive functioning and eventually screen for potential pharmacological treatment for the restoration of memory in patients with either brain injury-induced or age-related degeneration. (3) More generally, we plan to develop methods to directly measure information processing in the small larval brain of zebrafish (with few stereotypic neuronal projections), possibly while performing a behavior, in order to improve our understanding of neural information processing.
Equipment
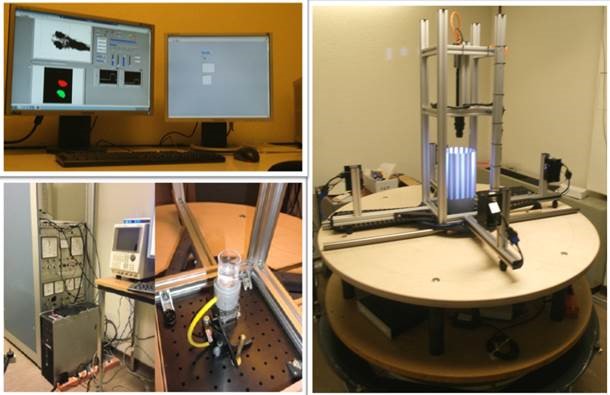
In humans, we mainly use non-invasive video-oculography to record eye behaviors in various psychophysical experiments including our recently invented on-line eye movement-feedback setup. In addition, we use fMRI and VEP for scanning our patients with specific eye movement disorders.
In zebrafish, we are equipped with a unique self-constructed horizontal fish turntable that allows us to precisely control and measure eye/body movements under different visual and/or vestibular stimuli. This state-of-the-art device enables us to conduct research in a variety of areas including sensorimotor control of eye movements, locomotion, body balance, motion perception. For surgical procedures, we are equipped with a clinical grade operating microscope for animals, which allows us to perform operations fast and precisely.
Selected publications
•Beppi C, Penner M, Straumann D, Bögli SY. Biomechanical induction of mild brain trauma in larval zebrafish: effects on visual startle reflex habituation. Brain Commun. 2023 Mar 15;5(2):fcad062. doi: 10.1093/braincomms/fcad062. PMID: 37006333; PMCID: PMC10065185.
•Beppi C, Penner M, Straumann D, Bögli SY. A non-invasive biomechanical model of mild TBI in larval zebrafish. PLoS One. 2022 May 27;17(5):e0268901. doi: 10.1371/journal.pone.0268901. PMID: 35622781; PMCID: PMC9140253.
•Beppi C, Beringer G, Straumann D, Bögli SY. Light-stimulus intensity modulates startle reflex habituation in larval zebrafish. Sci Rep. 2021 Nov 17;11(1):22410. doi: 10.1038/s41598-021-00535-9. PMID: 34789729; PMCID: PMC8599482.
•Beppi C, Ribeiro Violante I, Scott G, Sandrone S. EEG, MEG and neuromodulatory approaches to explore cognition: Current status and future directions. Brain Cogn. 2021 Mar;148:105677. doi: 10.1016/j.bandc.2020.105677. Epub 2021 Jan 21. PMID: 33486194.
•Beppi C, Straumann D, Bögli SY. A model-based quantification of startle reflex habituation in larval zebrafish. Sci Rep. 2021 Jan 12;11(1):846. doi: 10.1038/s41598-020-79923-6. Erratum in: Sci Rep. 2021 Apr 8;11(1):8166. PMID: 33436805; PMCID: PMC7804396.
•Beppi C, Violante IR, Hampshire A, Grossman N, Sandrone S. Patterns of Focal- and Large-Scale Synchronization in Cognitive Control and Inhibition: A Review. Front Hum Neurosci. 2020 Jun 25;14:196. doi: 10.3389/fnhum.2020.00196. PMID: 32670035; PMCID: PMC7330107.
•Lin TF, Gerth-Kahlert C, Hanson JVM, Straumann D, Huang MY-Y*. (2018) Spontaneous Nystagmus in the Dark in an Infantile Nystagmus Patient May Represent Negative Optokinetic Afternystagmus. Front. Neurol. 9:151. doi: 10.3389/fneur.2018.00151
• Bögli SY, Afthinos M, Huang MY*. Effect of gabapentin/memantine on the infantile nystagmus syndrome in the zebrafish model: implications for the therapy of ocular motor diseases. Invest Ophthalmol Vis Sci. 2017 June; 58(7):3149-57.
• Bögli SY, Huang MY*. Spontaneous alternation behavior in larval zebrafish. J Exp Biol. 2016 Nov 3. pii: jeb.149336.
• Chen CC, Bockisch CJ, Straumann D, Huang MY. Saccadic and postsaccadic disconjugacy in zebrafish larvae suggests independent eye movement control. Front Syst Neurosci. 2016 Oct; doi: 10.3389/fnsys.2016.00080.
• Bögli SY, Afthinos M, Bertolini G, Straumann D, Huang MY*. Unravelling stimulus direction dependency of visual acuity in larval zebrafish by consistent eye displacements upon optokinetic stimulation. Invest Ophthalmol Vis Sci. 2016 Apr; 57(4): 1721-7.
• Huber-Reggi SP, Mueller K, Straumann D, Huang MY, Neuhauss SC. Individual zebrafish larvae with infantile nystagmus syndrome display multiple nystagmus waveforms, which are influenced by viewing conditions. Invest Ophthalmol Vis Sci. 2014 May; doi: 10.1167iovs.13-13576.
• Chen CC, Huang MY, Weber KP, Straumann D, Bockisch CJ. Afternystagmus in darkness after suppression of optokinetic nystagmus: an ocular motor response to a motion aftereffect driven by retinal afterimages. Exp Brain Res. 2014 May; doi.org/10.1007/s00221-014-3971-4.
• Chen CC, Bockisch CJ, Olasagasti I, Weber KP, Straumann D, Huang MY*. Positive or negative feedback of optokinetic signals: Degree of the misrouted optic flow determines system dynamics of human ocular motor behavior. Invest Ophthalmol Vis Sci. 2014 Apr; 55(4): 2297–306.
• Chen CC, Bockisch CJ, Olasagasti I, Bertolini G, Weber KP, Neuhauss SCF, Straumann D, Huang MY*. Velocity storage mechanism in zebrafish larvae. J Physiol. 2014 Jan; 592(Pt 1):203-14.
• Huber-Reggi SP, Chen CC, Grimm L, Straumann D, Neuhauss SC, Huang MY*. Severity of infantile nystagmus syndrome-like ocular motor phenotype is linked to the extent of the underlying optic nerve projection defect in zebrafish belladonna mutant. J Neurosci. 2012 Dec; 32(50): 18079–86.
• Traber GL, Chen CC, Huang YY, Spoor M, Roos J, Frens MA, Straumann D, Grimm C. Albino mice as an animal model for infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2012 Aug; 53(9): 5737–47.
• Huang YY, Haug MF, Gesemann M, Neuhauss SCF. Novel expression patterns of metabotropic glutamate receptor 6 in the zebrafish nervous system. PLoS One. 2012 Apr; 7(4): e35256.
• Huang MY*, Chen CC, Huber-Reggi SP, Neuhauss SC, Straumann D. Comparison of infantile nystagmus syndrome in achiasmatic zebrafish and humans. Ann. N.Y. Acad. Sci. 2011 Sep; 1233: 285–91.
• Maurer CM, Huang YY, Neuhauss SC. Application of zebrafish oculomotor behavior to model human disorders. Rev. NeuroSci. 2011 Feb; 22(1): 5–16. (Review).
• Huang YY*, Tschopp M, Straumann D, Neuhauss SC. Vestibular deficits do not underlie looping behavior in achiasmatic fish. Commun Integr Biol. 2010 Jul; 3(4): 379–81.
• Huang YY, Tschopp M, Neuhauss SC. Illusionary self-motion perception in zebrafish. PLoS One. 2009 Aug 12;4(8):e6550.
• Huang YY, Neuhauss SC. The optokinetic response in zebrafish and its applications. Front Biosci. 2008 Jan 1;13:1899-916. (Review).
• Huang YY, Hedinger P, Neuhauss SC. Re: An oculomotor phenotype in question. J Neurosci. 2007 May 7; eLetters.
• Huang YY, Rinner O, Hedinger P, Liu SC, Neuhauss SC. Oculomotor instabilities in zebrafish mutant belladonna: a behavioral model for congenital nystagmus caused by axonal misrouting. J Neurosci. 2006 Sep 27;26(39):9873-80.
Funding
Swiss National Science Foundation, Betty and David Koetser Foundation for Brain research, Dr. Dabbous-Foundation
